In order to increase Hard Disk storage capacity, manufacturers have to store bits in ever-smaller clusters of atoms. Constantly pushing against their own their own manufacturing limits, manufacturers are finally one step away from reaching the physical limit of current technology and manufacturing techniques. As electronics shrink, electrical, magnetic, and even quantum interference begin to dominate, and it becomes ever more difficult to maintain and detect the values of stored bits.
To avoid that, scientists have started to explore the possibility of storing information in the chemical structure of single molecules. A team of European researchers reported a new approach to single-molecule storage that may bring these devices closer to stepping out of the lab.
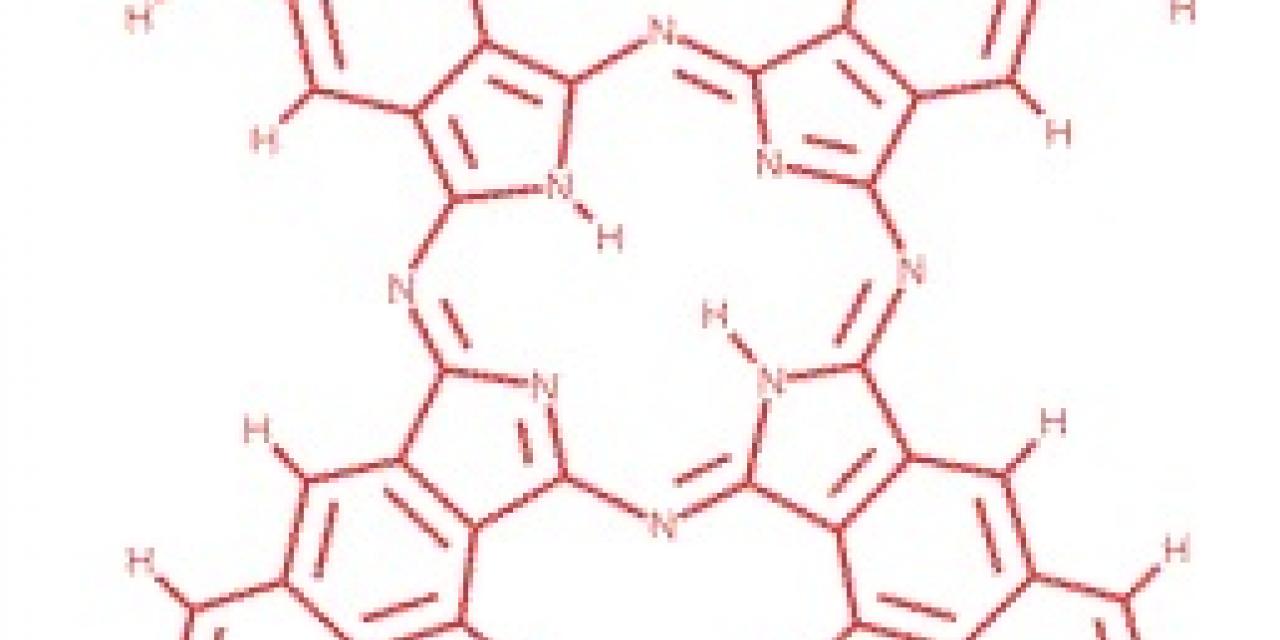
At the heart of the new technique is naphthalocyanine, a cross-shaped molecule consisting of a number of interlocking ringed structures. At the center of the cross, four nitrogen atoms face inward; two of those nitrogens, located opposite each other, are bonded to hydrogen atoms. The key fact is that it doesn't matter which two. There are two equally stable conformations of the molecule, termed tautomers, that differ only in the location of these hydrogen atoms.
In the new technique, naphthalocyanine molecules are layered on an insulating surface and chilled to five degrees above absolute zero then a scanning-tunneling microscope would be used detect the axis of the molecule that included the two hydrogens. By manipulating the current tunneling out of the microscope's tip, the hydrogen atoms swap locations. Because of the interlinked chemical structure of naphthalocyanine, energy pumped anywhere into the rings was able to induce this "tautomerization". In fact, putting energy into the end of one of the molecule's arms was the most efficient way of moving the hydrogens to a different location. Overall, the researchers claim that they can accurately set the state of these molecular bits 90 percent of the time.
Researchers then used the tip of the tunneling microscope to push several naphthalocyanine molecules close enough that the electrons in their ringed structures formed linked orbitals. Depending on where current is injected in the rings, different members of the structure can be selectively switched. They suggest that similar arrangements might also either allow the coordinated switching of a number of atomic bits, or enable the state of one bit to influence the response of its neighbors.
Clearly, this technology, which operates at near absolute zero and requires a tunneling microscope, is not ready for commercial use yet. Still, as the authors suggest, the basic approach is far more likely to produce usable technology than most of the other approaches currently being researched.



